The body’s response to COVID-19 infection can be a dangerous inflammatory overreaction known as a “cytokine storm,” that can lead to acute lung injury, fibrosis, multiple organ failure and death.1-4 Controlling the cytokine storm triggered by COVID-19 is critical to lowering mortality, especially in the elderly and those with chronic conditions such as diabetes, acute respiratory distress syndrome, hypertension and cardiovascular disease.2
Noveome believes that its lead biologic drug candidate, ST266, has the potential to prevent or significantly reduce the life-threatening cytokine storm observed in severely ill COVID-19 patients. ST266 has been shown to be safe and effective in numerous preclinical studies.
For example, preclinical studies in an acute systemic inflammation model demonstrated that ST266 was able to significantly reduce multiple inflammatory cytokines when administered systemically. The data from other preclinical studies demonstrate that ST266 can significantly reduce the cytokine-mediated inflammatory response in several indications.
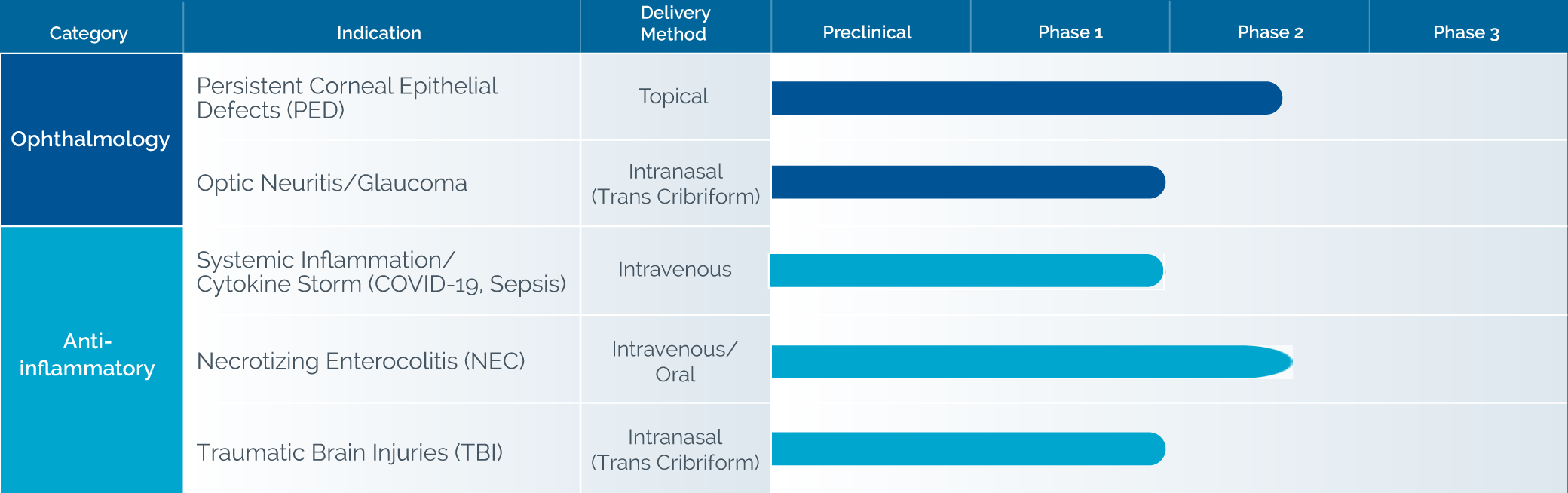
In clinical trials, 253 patients have been administered ST266 by topical dermal, topical oral, topical ocular or targeted intranasal delivery with no reported drug-related serious adverse events.
In clinical studies, topically applied ST266’s anti-inflammatory and healing activity has been demonstrated in UV light burns, radiation burns, gingivitis, and was shown to be beneficial in healing Persistent Corneal Epithelial Defects, and plans to initiate a Phase 2b trial by the end of 2021.
Allogeneic mesenchymal stem cells (MSCs) were shown to improve functional outcomes in seven treated patients with severe COVID-19 pneumonia.5 However, this type of cell therapy is associated with numerous safety and practical hurdles, especially during an emergency pandemic.
Noveome’s ST266 presents a unique opportunity to administer a multi-targeted COVID-19 cytokine storm countermeasure with many of the advantages of cell therapy, but without the cells or the associated risks.6-11 In addition to COVID-19, ST266 has the potential to treat other severe, acute systemic inflammatory responses and resultant multiple organ injuries caused by other inflammatory conditions such as sepsis. In March, 2021 Noveome initiated a Phase I clinical trial to examine the safety of intravenously delivered ST266 in COVID-19 patients. The first patient was dosed in June, 2021.
1. Tisoncik, J. R. et al. Into the Eye of the Cytokine Storm. Microbiol. Mol. Biol. Rev. (2012). doi:10.1128/mmbr.05015-11
2. Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (2020). doi:10.1016/S0140-6736(20)30183-5
3. Rubenfeld, G. D. et al. Incidence and outcomes of acute lung injury. N. Engl. J. Med. (2005). doi:10.1056/NEJMoa050333
4. McDermott, J. E. et al. Conserved host response to highly pathogenic avian influenza virus infection in human cell culture, mouse and macaque model systems. BMC Syst. Biol. (2011). doi:10.1186/1752-0509-5-190
5 Leng, Z. et al. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. ChinaXiv (2020).
6. Deng-Bryant, Y. et al. Long-term administration of amnion-derived cellular cytokine suspension promotes functional recovery in a model of penetrating ballistic-like brain injury. J. Trauma Acute Care Surg. 73, S156-64 (2012).
7. Deng-Bryant, Y. et al. Treatment with amnion-derived cellular cytokine solution (ACCS) induces persistent motor improvement and ameliorates neuroinflammation in a rat model of penetrating ballistic-like brain injury. Restor. Neurol. Neurosci. 33, 189–203 (2015).
8. Grinblat, G. A. et al. RGC neuroprotection following optic nerve trauma mediated by intranasal delivery of amnion cell secretome. Investig. Ophthalmol. Vis. Sci. 59, 2470–2477 (2018).
9. Khan, R. S., Dine, K., Wessel, H., Brown, L. & Shindler, K. S. Effects of Varying Intranasal Treatment Regimens in ST266-Mediated Retinal Ganglion Cell Neuroprotection. J. Neuro-Ophthalmology (2019). doi:10.1097/WNO.0000000000000760
10. Brown, L. et al. ST266 Amnion Cell-derived Cytokine Secretomefor Treatment of Hemorrhagic Shock and Trauma. MHSRS-19-02489 (2019).
11. Khan, R. S. et al. Intranasal Delivery of A Novel Amnion Cell Secretome Prevents Neuronal Damage and Preserves Function In A Mouse Multiple Sclerosis Model. Sci. Rep. 7, 41768 (2017).